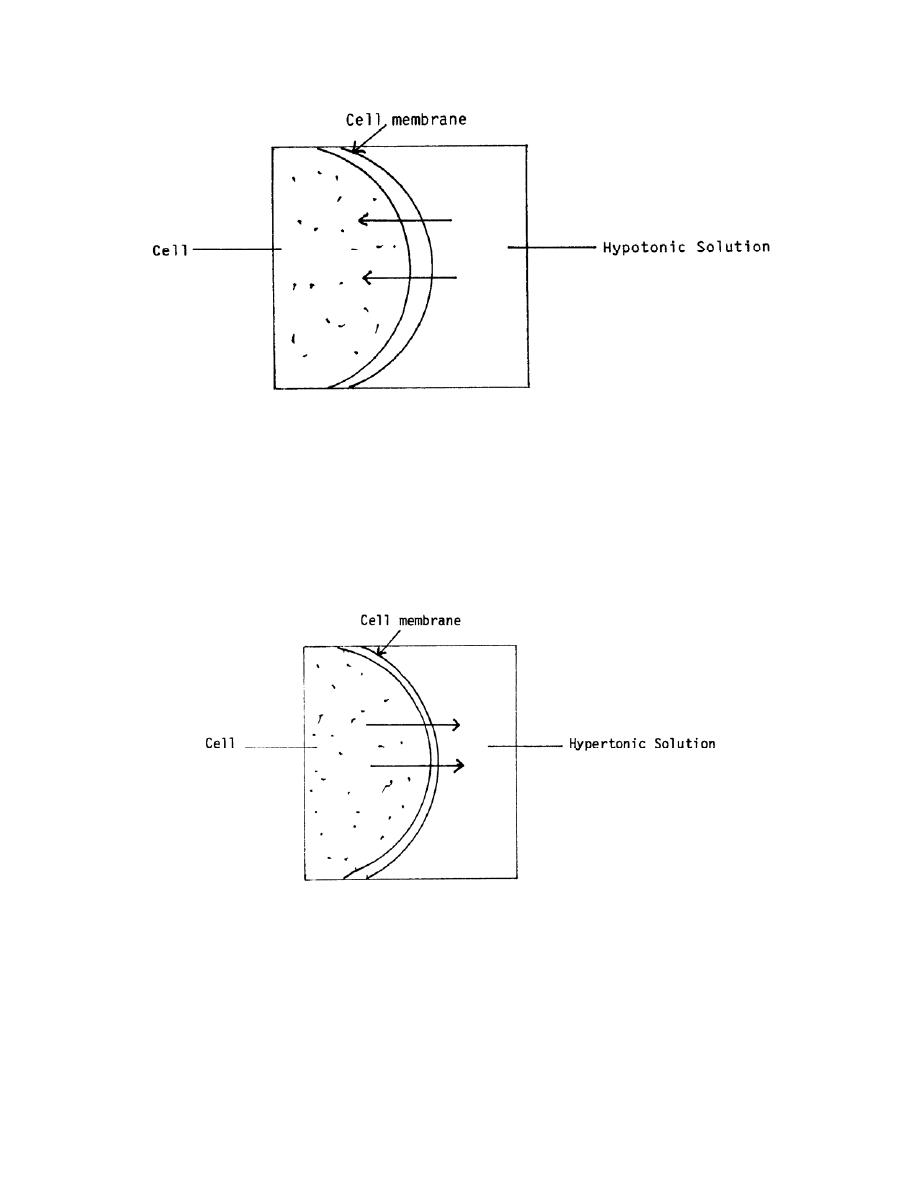
Figure 2-4. Hypotonic solution.
b. Hypertonic Solutions. A hypertonic solution is one that has greater tonicity
than the fluid within the body's cells. When this type of fluid is injected, it causes the
cells to lose fluid into the surrounding spaces. If too much hypertonic solution is
injected, the cells will shrink and shrivel. The cells become irritated, and this will
probably cause pain at the site of administration. Examples of hypertonic solutions are
hyperalimentation solutions and 10 percent dextrose solution. This movement is shown
in figure 2-5.
Figure 2-5. Hypertonic solution.
c. Isotonic Solutions. An isotonic solution has the same tonicity as that of
body fluids. When this type of fluid is injected, fluids travel equally in both directions.
Injection of an isotonic fluid causes no cell irritation to occur. Examples of isotonic fluids
are 0.9 percent sodium chloride solution and lactated Ringer's solution. This
movement is shown in figure 2-6.
MD0564
2-8




 Previous Page
Previous Page
